Proven Safety Profile1
Treatment-emergent adverse events (TEAEs) occurring in the RI and the RW periods of the RHAPSODY study in ≥5% of patients
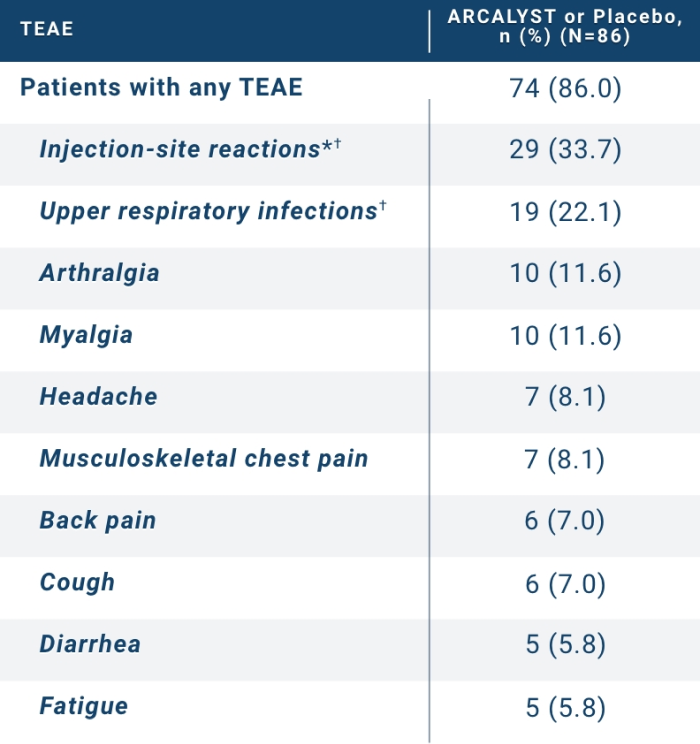
74 (86.0)
29 (33.7)
19 (22.1)
10 (11.6)
10 (11.6)
7 (8.1)
7 (8.1)
6 (7.0)
6 (7.0)
5 (5.8)
5 (5.8)
During the RI/RW periods (n=86):
- No adverse events (AEs) led to death
- Four AEs led to treatment discontinuation
- Five serious AEs (SAEs) occurred: 1 during the RI period (stroke due to carotid artery dissection), 1 in the ARCALYST treatment arm (squamous cell carcinoma), 1 in the placebo arm (cardiac flutter), and 2 in the placebo arm following ARCALYST bailout (pyrexia and ileus)
During the LTE period (n=74):
- 62 (83.8%) of patients experienced TEAEs
- Six patients experienced SAEs: (1. pneumothorax; 2. acute endocarditis, aortic valve disease, acute myocardial infarction, pericarditis; 3. transient ischemic attack, coronavirus infection; 4. worsening of aortic insufficiency; 5. pneumonia, pneumonia viral [COVID‐19]; and 6. left ventricular failure, hip fracture, bile duct stone, cardiac device malfunction)
- Three AEs led to treatment discontinuation and no AEs led to death
- TEAEs of interest: upper respiratory infections and injection-site reactions were reported at 16.2% and 6.8%, respectively
Reference: 1. Data on file. Kiniksa Pharmaceuticals.

