Break the Cycle of Recurrent Pericarditis
to relieve pain, resolve inflammation, and prevent recurrences1,2
ARCALYST breaks the self-perpetuating cycle of IL-1-mediated autoinflammation by blocking IL-1 signaling, thereby relieving pain, resolving inflammation, and preventing recurrences.

In the Rhapsody RW period and LTE, ARCALYST was proven to prevent recurrences
In the RW period (primary efficacy end point)*:
ARCALYST significantly reduced the risk of pericarditis recurrence.1,5
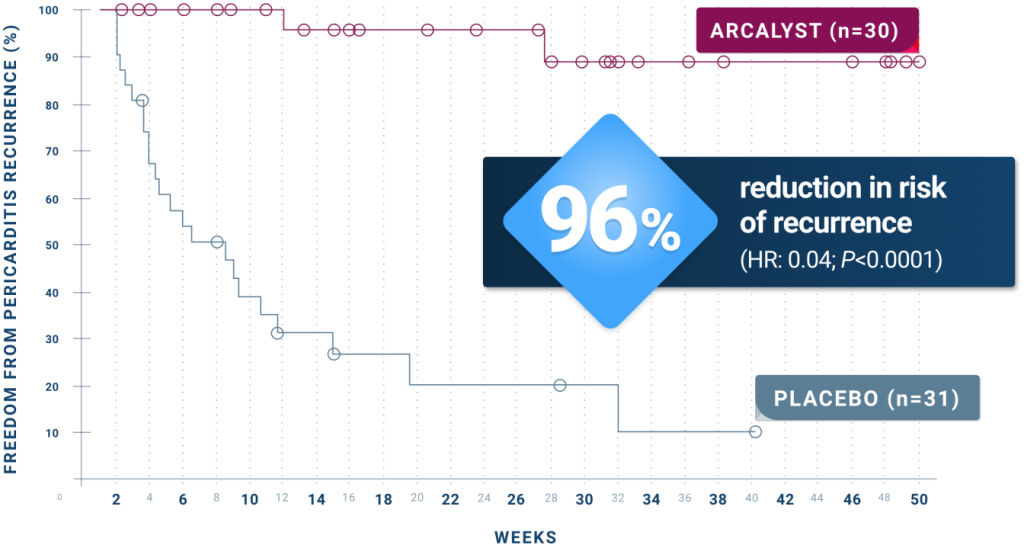
The median time to recurrence on ARCALYST could not be estimated due to the low numbers of recurrence:
- 7% (2 of 30) of patients treated with ARCALYST experienced a recurrence
- The 2 pericarditis recurrences occurred during temporary treatment interruptions of 1 to 3 weekly ARCALYST doses
The median time to recurrence on placebo was 8.6 weeks (95% CI: 4.0, 11.7):
- 74% (23 of 31) of patients treated with placebo experienced a recurrence by the time the event-driven RW portion of the trial was closed
In the LTE period
Long-term Prevention While on Treatment
99% (74 of 75) of eligible patients chose to remain in the study to be treated with open-label ARCALYST for up to an additional 24 months.6†
- Participants in the LTE were treated with ARCALYST for a median of ~24 months from RI baseline
- The median duration of ARCALYST treatment in the base study was 9 months
ARCALYST continued to significantly reduce recurrences up to the 18-month decision milestone:
ARCALYST continued to significantly reduce recurrences past the 18-month decision milestone6§
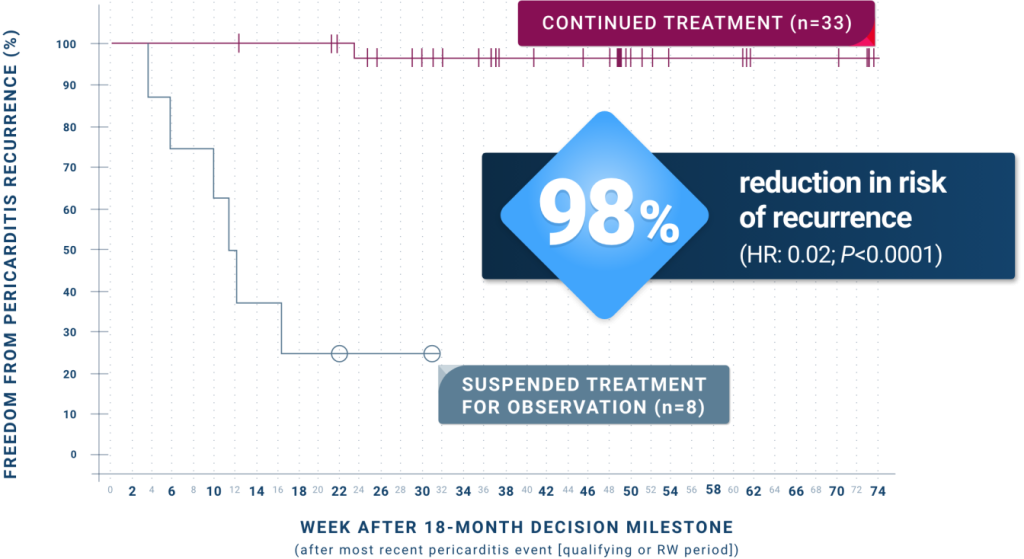
Patients received a median of ~2 years of ARCALYST treatment from RI baseline.
- Recurrence rate was 3% (1/33) in patients who continued ARCALYST treatment vs 75% (6/8) in patients who suspended treatment for observation
- The only recurrence in the group treated with ARCALYST beyond the 18-month decision milestone was associated with a treatment interruption of 4 weeks
- The median time to recurrence in patients who suspended ARCALYST treatment for observation was 11.8 weeks, and the median time to recurrence in patients who continued treatment was not estimable
ARCALYST Rapidly Relieved Pain and Resolved Inflammation
In the RI period (secondary efficacy end point):

In the RW period (secondary efficacy end points assessed at week 16):
Patients reported

ARCALYST Was Steroid Sparing

References: 1. ARCALYST. Package insert. Kiniksa Pharmaceuticals. 2. Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012; 11(8): 633-652. doi: 10.1038/nrd 3800 3. Chiabrando JG, Bonaventura A, Vecchié A, et al. Management of acute and recurrent pericarditis. J Am Coll Cardiol. 2020; 75(1): 76-92. 4. Klein A, Cremer P, Kontzias A, et al. Clinical burden and unmet need in recurrent pericarditis: a systematic literature review. Cardiol Rev. 2022; 30(2): 59-69. doi: 10.1097/ CRD.0000000000000356 5. Klein AL, Imazio M, Cremer P, et al. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N Engl J Med. 2021; 384(1): 31-41. 6. Imazio M, Klein AL, Brucato A, et al. Sustained Pericarditis Recurrence Risk Reduction With Long-Term Rilonacept. J Am Heart Assoc. 2024; 13(6): e032516. doi: 10.1161/ JAHA.123.032516.


