WHAT ARE CAPS?
CAPS are a group of rare, hereditary autoinflammatory disorders.1,2 They are inherited in an autosomal dominant pattern, which means that a mutation in 1 copy of the gene is enough to cause the disorder.2,3
- Both males and females are equally affected.4
- The estimated incidence in the United States is 1 to 2 cases per million people.2
- CAPS are characterized by lifelong recurrent symptoms of rash, fever/chills, joint pain, eye redness/pain, and fatigue.4
- The symptoms of CAPS may be challenging to diagnose.
- The diagnosis may be delayed because CAPS are rare and the symptoms often resemble those of other disorders.
- Intermittent symptom flares may be triggered anytime by cooling temperatures, stress, exercise, or unidentified causes. Some patients may try to reduce their symptoms by avoiding things that trigger the disease.

ARCALYST is a targeted inhibitor of IL-1, the key driver of inflammation in Cryopyrin-Associated Periodic Syndromes (CAPS). It is approved for the treatment of CAPS, including Familial Cold Auto-inflammatory Syndrome (FCAS), and Muckle-Wells Syndrome (MWS) in adults and children 12 and older.4
DOSING AND ADMINISTRATION
ARCALYST is a patient-administered once-weekly subcutaneous therapy.4
Adults
(18 years and older)
Adolescents
(12 to 17 years)
The loading dose of ARCALYST should be performed under the supervision of a healthcare professional.
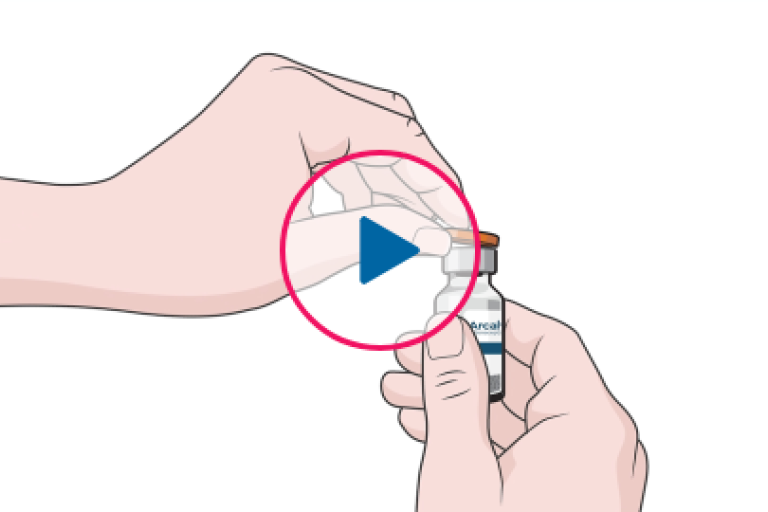
STEP BY STEP ADMINISTRATION
ARCALYST reconstitution and injection follow a step-by-step process.4

ARCALYST is supplied in sterile, single-use, 20-mL glass vials.1
- Each vial contains 220 mg rilonacept, a sterile, white to off-white, preservative-free, lyophilized powder
- Reconstitution with 2.3 mL of Sterile Water for Injection is required prior to subcutaneous administration of the drug
- The reconstituted ARCALYST is a viscous, clear, colorless to pale yellow, free from particulates, 80-mg/mL solution
INITIATING TREATMENT
Steps to initiate ARCALYST treatment
- Ensure your patient’s vaccination history is up to date, including their pneumonia and flu vaccines.
- The Enrollment Form will be provided by your Kiniksa Sales Specialist or is available for download below.
- Fax completed Enrollment Form to Kiniksa OneConnect™ at (781) 609-7826.
- Your patient will be contacted by Kiniksa OneConnect™ to help arrange delivery from select specialty pharmacies.
- Kiniksa OneConnect™ will work with your patients to set up one-on-one injection training sessions with either virtual support from an ARCALYST Clinical Educator or a combination of virtual/in-person training support that includes virtual support from an ARCALYST Clinical Educator and in-person support from a healthcare professional.
Kiniksa OneConnect can help your patients with their treatment needs.
TREATMENT RESOURCES
There may be several steps to obtaining approval for your patient’s treatment with ARCALYST. The downloadable resources below were developed to help simplify this process by providing information about gaining access to ARCALYST, understanding reimbursement claims, and working with a specialty pharmacy.
Access
The following downloadable resources can help guide you through the steps to product access.
Financial Assistance
Resources for information about access to ARCALYST and navigating the reimbursement process .
KINIKSA ONECONNECT™
Kiniksa OneConnect™ is a support program made up of a team of experienced individuals, known as Patient Access Leads, with knowledge of insurance plans and healthcare networks.
Patient Access Leads
Once enrolled in Kiniksa OneConnect™, your patient will be paired with a dedicated Patient Access Lead who will work to provide personalized one-on-one support throughout your patient's entire treatment experience.
ARCALYST Clinical Educators
ARCALYST Clinical Educators can conduct sessions to help provide your patients with training on the injection process.
Beginning treatment
By helping to navigate your patients through their insurance coverage and partnering with your practice, we make getting your patient on treatment a seamless experience in a variety of ways:
- We coordinate, verify, and explain the benefits verification process
- We assist with the prior authorization and appeals processes if required
- When it's time for your patients to begin treatment, we help coordinate delivery of your patient’s therapy




Help your patients learn more about our services.
References: 1. Kastner DL. Familial Mediterranean fever and other hereditary autoinflammatory diseases. In: Jameson JL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed.: McGraw-Hill Education; 2018:2610-2614. 2. Kuemmerle-Deschner JB. CAPS — pathogenesis, presentation and treatment of an autoinflammatory disease. Semin Immunopathol. 2015;37(4):377-385. 3. Autosomal dominant inheritance. National Cancer Institute Dictionary of Genetics Terms. Accessed February 23, 2021. https://www.cancer.gov/publications/dictionaries/genetics-dictionary/def/autosomal-dominant-inheritance. 4. ARCALYST. Package insert. Kiniksa Pharmaceuticals.